M. DMD medicine
Rare genetic diseases in children Development of medicine for Duchenne Muscular Dystrophy (DMD)
-
 Efficacy Test
Efficacy Test
-
 Toxicity Test
Toxicity Test
-
 Clinical Test
Clinical Test
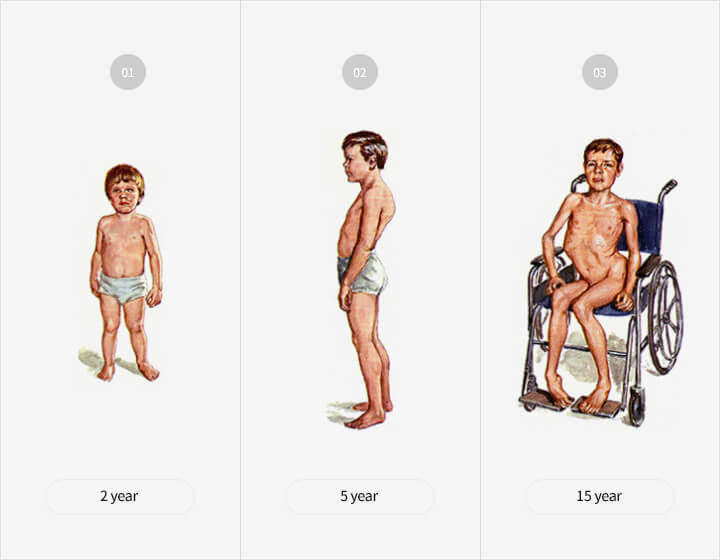
Characteristics of the disease
- 80 to 85% of all patients with muscular dystrophy are
DMD patients - Ratio of nearly 1 in 3,500 newborn boys
- Symptoms such as posture deformation which
typically begin at the age of 3-4, leading to gradual
atrophy of muscles
→ Most die in their early 20s when respiratory
muscles become too weak - Currently, a full recovery is not possible due to the
absence of medicine, which increases the necessity
of developing a new drug
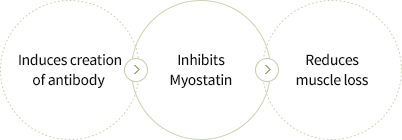
- Selected Myostatin, an inhibitor of muscle creation, as the target antigen
Source : https://auroshealthcare.wordpress.com/
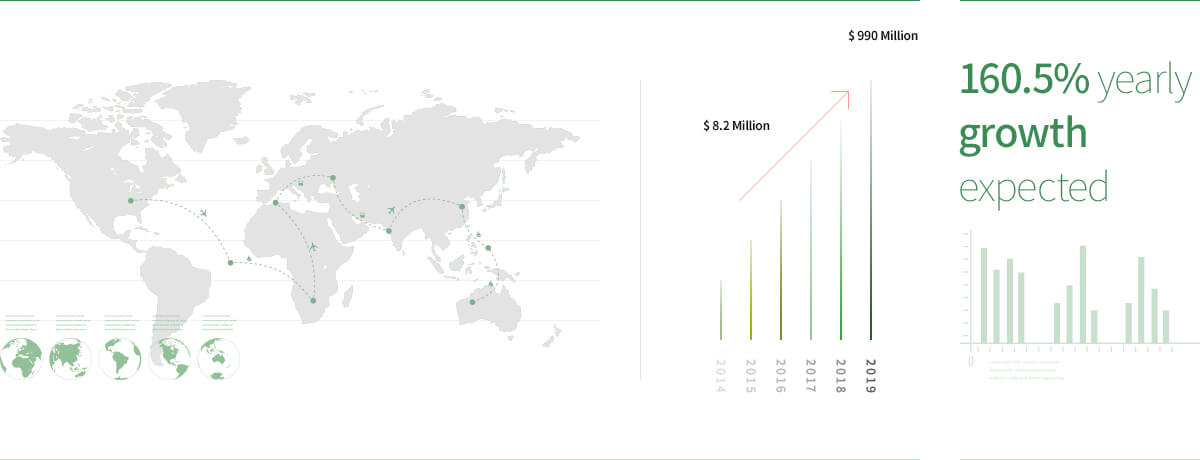
Global market value
Status
Approved with the Orphan Drug Designation (ODD) by the U.S. FDA in February 2018 → Increased possibility for acquisition of PRV
Orphan Drug Designation benefits
- Provision of research subsidies during the clinical test period
- Tax deductions for clinical test expenses
- Cost exemption for the review of new drug approval applications
- Permission of conditional sales after Clinical Phase 2
- Assignment of 7-year, exclusive marketing rights after permission is granted for marketing
- [SAMSUNG MEDICAL CENTER] Signed an agreement for joint R&D in January 2012
- Selected for and executed the assignment to develop advanced medical technology given by Ministry of Health and Welfare (2015 - 2018)
Strategy
- Complete Clinical Phase 2 · Acquire permission for marketing from U.S. FDA
- Secure additional technical partners → Establish a joint venture with a local partner or proceed with licensing out
- Acquire PRV
- A voucher received when developing an innovative new drug for rare diseases designated by the U.S. FDA :
The period for approval of the development company's other medicines reduced to 6 months
→ Period of revenue may be extended, which leads to increases in sales - Possible to use or sell directly : The current sales value of PRV sits at over KRW 210 billion on average

· Status of PRV acquisition in relation to DMD disease
| Year | Company | Drug | Comments |
|---|---|---|---|
| 2017 |  |
Emflaza(deflazacort) | FDA Press release |
| 2016 |  |
Exondys51(eteplirsen) | |
| Sarepta has sold PRV to Gilead for $125m |  |
||
Source : http://priorityreviewvoucher.org